|
I have a new paper out in the journal Ecology-- Nectar chemistry mediates behavior of parasitized bees: consequences for plant fitness. This is dissertation research conducted with my mentor at Dartmouth College, Becky Irwin (now at North Carolina State University), and Deane Bowers of University of Colorado, Boulder. In this work, we measured iridoid glycoside chemistry of turtlehead flowers at multiple sites around northern Vermont, finding a good deal of variation among plants. I had previously found in lab experiments that iridoid glycosides can benefit bees by reducing parasite infections in their guts, and because the chemicals have an intensely bitter flavor (at least to us!), we wondered whether bees would seek out flowers with the highest concentrations of these compounds when parasitized. We created arrays of flowers with experimentally altered nectar chemistry, and recorded wild bee foraging behavior on these flowers, later measuring parasite load of each bee. We found that parasitized bumble bees did indeed respond to iridoid glycoside chemistry, foraging longer at flowers with highest chemical concentrations and returning for second meals with greater frequency to those flowers. By contrast, bees without parasite infections didn't make any distinctions between flowers of different chemical concentrations. In a second experiment, we found that those extra bitter flowers had higher reproductive success, exporting more pollen on the backs of bees to other flowers.
All of this suggests that the secondary metabolite chemicals naturally present in nectar can function as attractants, and that pollinators might self-medicate with floral products when challenged by natural enemies. We previously got some press about this work from Discover Magazine and Science Daily. You can see the abstract--and cover image of this interaction taking place--here. And here's a copy of the paper if you want to read it. Richardson, L.L., R.E. Irwin and M.D. Bowers. 2016. Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 97(2): 325-337.
1 Comment
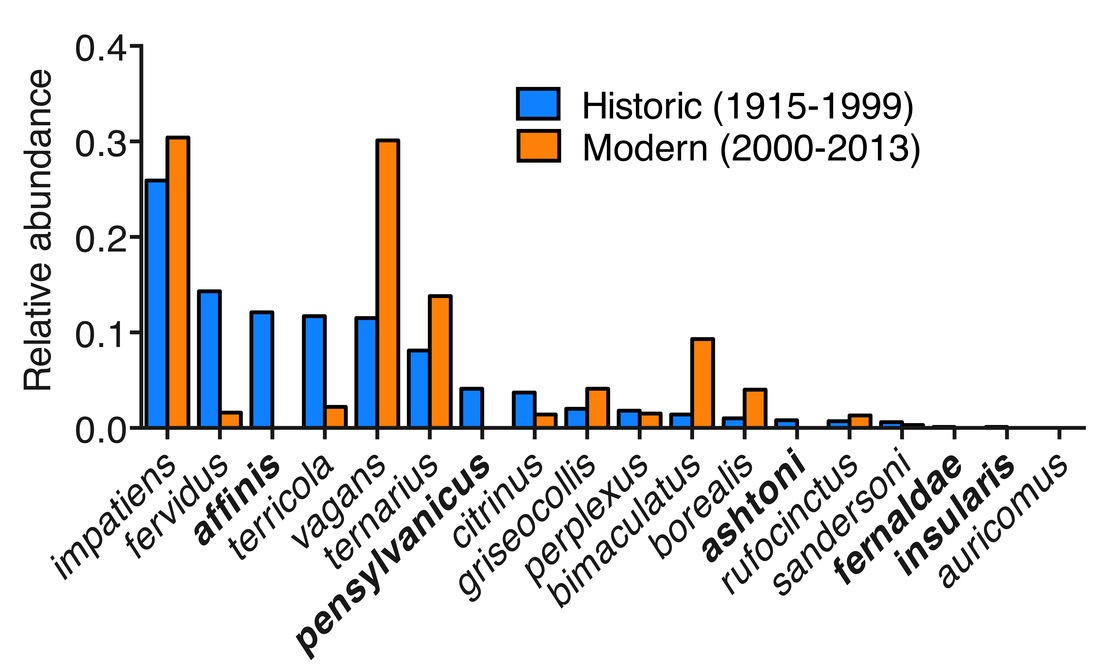
With co-authors at the Vermont Center for Ecostudies (VCE) I'm writing a manuscript on changes in bumble bee species distribution in Vermont over the last century. We compared historic (<1999) museum specimen data to modern (2000-2014) collections, including a two year citizen science project in which VCE volunteers collected more than 10,000 new records of bumble bee species distribution around the state.
What did we find? Eighteen species of bumble bee are native to Vermont, and five of these, shown in bold in the figure below, have apparently been extirpated. A number of other species have declined in relative abundance between the two periods also. (Relative abundance is a coarse metric for comparison of a single species' contribution to a larger dataset.) For example, Bombus fervidus and B. terricola, important pollinators of wild and cultivated plants in our area, have greatly declined in relative abundance between the two time periods. Others, such as B. vagans, are much more common now than in the past, but we do not know the degree to which these species have assumed the ecological niches of those we have lost. This project allowed species status assessments that contributed to listing of two species as Endangered in Vermont, and another as Threatened. Much more on this project to come! 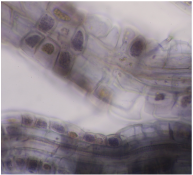 Many wild plants form below-ground associations with fungi, exchanging the products of photosynthesis for increased access to soil nutrients and water, as well as a range of other benefits. Mycorrhizal fungi are often present in agricultural soils also, but we usually don't account for them in farm management. But if plants can benefit from these associations, shouldn't we consider the potential that they affect crop yield? I'm in the middle of my first foray into visualizing one type of these fungi, the ericoid mycorrhizal species found on blueberry roots. In the picture here, you can see coils of blue-gray fungal hyphae inside the cortical cells of blueberry roots from a farm in northern Vermont. The color is not natural: to visualize these structures you have to bleach out the root's color, then stain the fungi. Why are these things worth visualizing? In an ongoing experiment (more on this soon), I have found that when I actively inoculate blueberry plants with ericoid mycorrhizal fungi, they behave differently, growing larger leaves and longer flowers, and becoming less appealing to herbivores. There is also some suggestion that inoculated plants make larger fruit, meaning that farmers could realize a long-term benefit from an investment in fungus at planting time.  I'll start here: this is Vaccinium corymbosum, the wild progenitor of cultivated highbush blueberry. I work on the multiple direct and indirect interactions between cultivated blueberry, ericoid mycorrhizal fungi and pollinating bees, but I think it's instructive to visit native populations of the plant occasionally to see how these interactions progress in the wild. And to pick the fruit! Blueberry is an interesting crop because efforts to domesticate the plant began only about 75 years ago. This is in contrast to the long periods of artificial selection most of our food plants have undergone. Here's a recent news piece on the domestication of highbush blueberry. |
AuthorEcologist at UVM's Gund Institute for Ecological Economics. Posts about plant-insect interactions, bees, parasites and life. Archives
March 2016
Categories |

 RSS Feed
RSS Feed
